Abstract
INTRODUCTION Cancer survivors are at elevated risk for developing type 2 diabetes mellitus, which decreases quality of life and increases mortality during cancer survivorship. Type 2 diabetes results from a combination of insulin resistance and decreased insulin secretion. Assessing pancreatic β-cell function by measuring insulin secretion dynamics in response to hyperglycemia can provide insight into the pathogenesis of type 2 diabetes. We investigated insulin secretion in patients with treated hematological malignancies and compared them to non-diabetic controls without cancer. We hypothesized that patients with hematological malignancies will have decreased insulin secretion compared to controls without cancer due to β-cell toxicity from previous treatment and chemotherapy.
METHODS We evaluated insulin secretion in 16 non-diabetic adult patients with treated hematological malignancies using a hyperglycemic clamp, which assesses pancreatic β-cell function by measuring insulin secretion in response to hyperglycemia (200 mg/dL) maintained by a dextrose infusion. Clamp outcomes included first phase (T0-10 minutes), second phase (T90-120 minutes), and maximal (L-arginine stimulated) insulin response. The insulin sensitivity index (ISI) and disposition index (DI, a measure of the β-cell function to compensate for insulin resistance) were calculated. Controls (n=32) matched for age, sex, race, and body mass index (BMI) were selected 2:1 from a database of 154 hyperglycemic clamps in participants without diabetes or cancer. Demographic characteristics and clamp outcomes were compared using χ2, Wilcoxon rank-sum test, or linear regression.
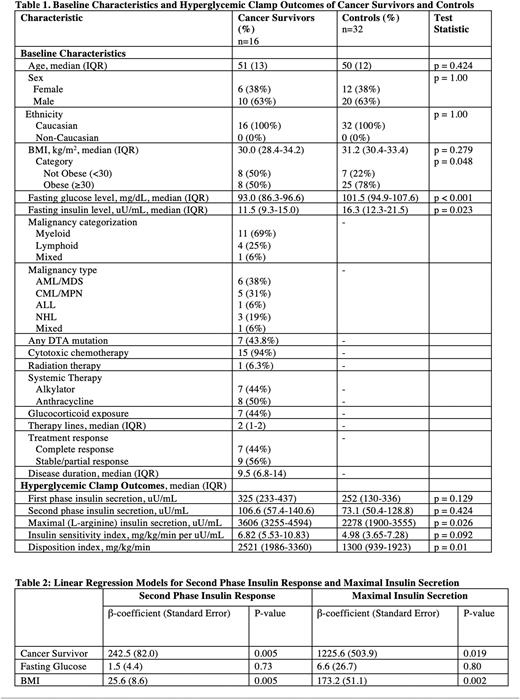
RESULTS Hyperglycemic clamps were performed in 48 individuals without diabetes (16 patients with treated hematological malignancies and 32 matched controls without cancer). Cancer survivors and controls without malignancy were well-matched for age, sex, race (Table 1). Myeloid malignancies were the most common diagnosis (n=11; 69%) with a median time from diagnosis to metabolic testing of 6 months. Treatment included cytotoxic chemotherapy (n=15; 94%), glucocorticoids (n=7; 44%), and radiation (n=1; 6%) with 7 (44%) patients achieving a remission prior to testing. Compared to controls, patients with malignancy had lower fasting glucose levels (93.0 mg/dL [IQR 86.3-96.6] vs 101.5 [IQR 94.9-107.6], p<0.001) and lower fasting insulin levels (11.5 uU/mL [IQR 9.3-15.0] vs 16.3 [IQR 12.3-21.5], p=0.023), suggesting greater insulin sensitivity within the cancer cohort. In univariable analysis, there were no significant differences between first and second phase insulin response between patients with or without treated hematological malignancies (Table 1). Cancer survivors had a higher maximal (L-arginine stimulated) insulin secretion (3606 uU/mL [IQR 3255-4594] vs 2278 [IQR 1900-3555], p=0.026) and a higher disposition index (2521 mg/kg/min [IQR 1986-3360] vs 1300 [IQR 939-1923], p=0.01) than in controls. Cancer survivors demonstrated a trend toward greater insulin sensitivity compared to controls (6.82 mg/kg/min per uU/mL [IQR 5.53-10.83] vs 4.98 [IQR 3.65-7.28], p=0.092). After adjustment for BMI and fasting glucose in linear regression models, both second phase (β=242.5, p=0.005) and maximal insulin secretion (β=1225.6, p=0.019) were greater in cancer survivors then in controls without cancer (Table 2).
CONCLUSION Cancer survivors had higher maximal phase insulin secretion and disposition index (a measure of β-cell function adjusted for insulin resistance) compared to non-diabetic controls without cancer. Our data suggests that cancer survivors have preserved β-cell function following treatment of their hematological malignancies, contrary to our hypothesis. Longitudinal follow-up is needed to determine factors that promote diabetes susceptibility in cancer survivors.
Disclosures
Byrne:Karyopharm: Research Funding; Taiho: Research Funding; CTI: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celularity, Concert: Consultancy, Other: DSMC. Sengsayadeth:Amgen: Research Funding. Dholaria:Pfizer: Research Funding; Orca Bio: Research Funding; Angiocrine: Research Funding; BMS: Research Funding; Jazz Pharmaceuticals: Consultancy; Takeda: Research Funding; Poseida: Research Funding; MEI Pharma: Research Funding; Janssen: Research Funding; BEAM Therapeutics: Consultancy; Gamida Cell: Consultancy; Vanderbilt University Medical Center: Current Employment; Wugen: Research Funding; Arivan: Consultancy; MJH Biosciences: Honoraria; Molecular Templates: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal